New Life for Challenging Wounds
Few medical problems are more complex or result in more difficult-to-heal cases than lower extremity wounds caused by the comorbidities of diabetes. In fact, there are more than 90,000 lower extremity amputation procedures performed on patients, including those with diabetes, in the U.S. annually. Limb preservation is our number one priority.
Historically, natural amniotic membranes have been successfully used for wound and reconstructive purposes since the early 20th century.
In examining the use of amniotic membrane (AM) grafts on patients with chronic venous leg ulcers, studies have yielded positive results.*
The Wonder of Amniotic Membrane
Revitalon is derived from the human placenta, an immunoprivileged environment steeped in growth factors and cytokines. In an allograft form, the complex membrane structure allows the patient’s native cells to thrive, even in chronic, non-healing wounds.
Optimal Tissue Processing
Minimally Processed – The graft passes USP <71> Sterility Testing and is processed and packaged using strict aseptic technique. No irradiation is used in order to preserve the natural integrity of the tissue.
Safety Assured – MTF, the nation’s leading tissue bank, procures the tissue for Revitalon. MTF has an exemplary safety record with comprehensive donor vetting, including screening for high-risk behaviors and conducting laboratory tests for a number of potential pathogens.
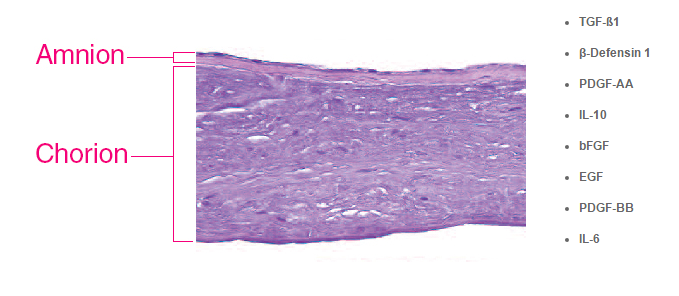
Growth Factors and Cytokines Shown to be Present in Revitalon Amniotic Membrane*
Case studies are available upon request.