Exclusive Gelling Technology Provides Powerful Yet Gentle Moisture Management
Chytoform™ Chitosan-based Gelling Technology
Opticell with Chytoform technology is the next generation of chronic wound care dressings. Chytoform gelling fiber is primarily composed of chitosan, a well-known biological material derived from crustacean shells. Chitosan’s unique chemistry, including a positive charge at physiological pH, has made it the center of much academic and clinical research(1-30).
Medline Opticell dressings are the first application of this advanced biological material for use in chronic wound care, but chitosan has been used in other healthcare applications (e.g., topical hemostat, treatment of surgical wounds and traumatic injuries and in dietary supplements) because of its unique properties.
When Opticell with Chytoform technology comes into contact with moisture, it transforms into a strong, absorbent and conformable gel that controls minor bleeding common in newly debrided wounds.
Main Benefits
Creates An Optimal Healing Environment
- Manages moisture to help promote autolytic debridement
- Optimizes wound contact with a highly conformable low profile
- Maintains wound coverage with Surface Area Memory (SAM)
Gentle Patient Care
- Prevents maceration by wicking fluid only vertically, not laterally
- Reduces dressing change frequency with exceptional absorbency
- Separates gently from the wound in one piece using advanced Cytoform technology
A Market Leader in Absorbency
A recent study compared Opticell’s absorptive capabilities to those of our main competitors.*
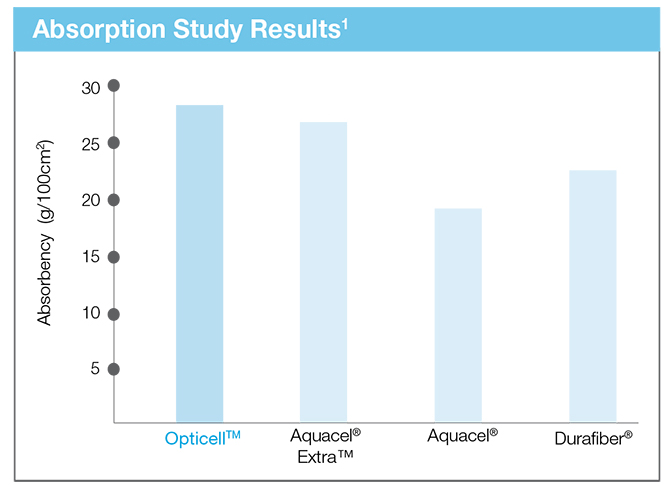
Reference: *Lab testing data on file.
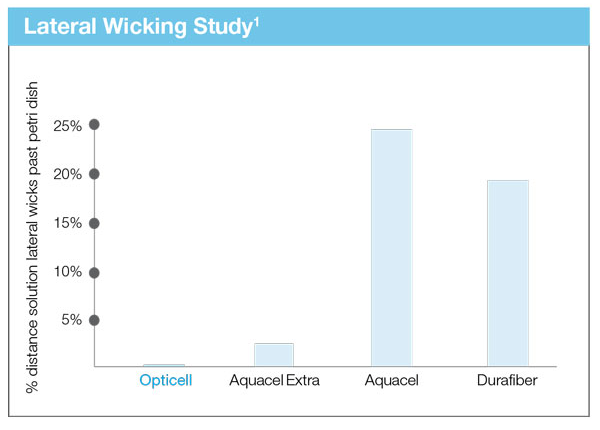
Helps to Prevent Maceration
Opticell’s Chytoform technology wicks fluid only vertically, not laterally. This reduces the risk of periwound maceration because wound fluid is not able to migrate across the dressing to reach this vulnerable skin.
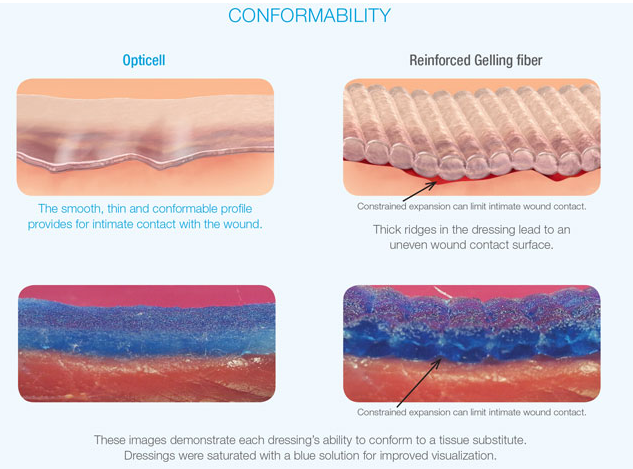
Conforming to the Wound
Opticell’s conformable and thin profile provides optimal contact with the wound. Other dressings with reinforced fibers, on the other hand, may exhibit a ridging effect that can limit wound contact.
References
- Lord MS, Cheng B, McCarthy SJ, Jung M, Whitelock JM. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 2011;32(28):6655-62.
- Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res 1997; 34: 21-8
- Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev 2001;52(2):105-15
- Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol 2010;56(3):290-9.
- Chung MJ, Park JK, Park YI. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int Immunophamacol. 12 (2): 453-9, 2012.
- Henryk Struszczyk: Alginate and Chitosan Fibers for Medical Uses. In Natural Fibers, Plastics and Composites Editors: Frederick T. Wallenberger, Norman E. Weston 2004, pp 95-104
- Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. European Polymer Journal [serial online]. April 2013;49(4):780-792.Available from: Academic Search Alumni Edition, Ipswich, MA. Accessed July 15, 2013.
- Madhally SV, Matthew HWT. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999: 20 1333-42
- Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose Metabolism and Pharmacokinetics in Man. Food and Chemical Toxicology 38 (Suppl. 2) (2000)
- Pillai CKS, Paul W, Sharma CP. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 2009; 34: 641-78
- Agboh OC, Qin Y. Chitin and chitosan fibers. Polym Adv Technol 1997; 8: 355-65
- Zhang X, Yang D, Nie J. Chitosan/polyethylene glycol diacrylate films as potential wound dressing material. Int J Biol Macromol 2008; 43: 456-62
- Okamoto Y, Kawakami K, Miyatake K, Morimoto M, Shigemasa Y, Minami S. Analgesic effects of chitin and chitosan. Carbohydrate Polymers [serial online]. August 15, 2002;49(3):249-252. Available from: Academic Search Alumni Edition, Ipswich, MA. Accessed July 15, 2013.
- Honglue T, Rui M, Chucheng L, Ziwei L, Tingting T. Quaternized Chitosan as an Antimicrobial Agent: Antimicrobial Activity, Mechanism of Action and Biomedical Applications in Orthopedics. International Journal Of Molecular Sciences [serial online]. January 2013;14(1):1854-1869. Available from: Academic Search Alumni Edition, Ipswich, MA. Accessed July 15, 2013.
- Rabea, Entsa, Mohamed E.-T. Badawy, Christian V. Stevens, Guy Smagghe, and Walter Steurbaut. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules [serial online]. December 2003; 4(6). Available from: Academic Search Alumni Edition, Ipswich, MA. Accessed July 15, 2013.
- Heinze T, Koschella A. Carboxymethyl Ethers of Cellulose and Starch – A Review Macromolecular Symposia. Special Issue: Cellulose and Cellulose Derivatives Volume 223, Issue 1, pages 13–40, March 2005
- Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg 2010;251(2):217-28.
- Dowling MB, Smith W, Balogh P, Duggan MJ, MacIntire IC, Harris E, Mesar T, Raghavan SR, King DR. Hydrophobically-modified chitosan foam: description and hemostatic efficacy. J Surg Res 2015;193(1):316-23.
- Kulling D, Vournakis JN, Woo S, Demcheva MV, Tagge DU, Rios G, Finkielsztein S, Hawes RH. Endoscopic injection of bleeding esophageal varices with a poly-N-acetyl glucosamine gel formulation in the canine portal hypertension model. Gastrointest Endosc 1999;49(6):764-71.
- Huang X, Sun Y, Nie J, Lu W, Yang L, Zhang Z, Yin H, Wang Z, Hu Q. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int J Biol Macromol 2015;75:322-9.
- Fischer TH, Connolly R, Thatte HS, Schwaitzberg SS. Comparison of structural and hemostatic properties of the poly-N-acetyl glucosamine Syvek Patch with products containing chitosan. Microsc Res Tech 2004;63(3):168-74.
- Ikeda Y, Young LH, Vournakis JN, Lefer AM. Vascular effects of poly-N-acetylglucosamine in isolated rat aortic rings. J Surg Res 2002;102(2):215-20.
- Chan MW, Schwaitzberg SD, Demcheva M, Vournakis J, Finkielsztein S, Connolly RJ. Comparison of poly-N-acetyl glucosamine (P-GlcNAc) with absorbable collagen (Actifoam), and fibrin sealant (Bolheal) for achieving hemostasis in a swine model of splenic hemorrhage. J Trauma 2000;48(3):454-7.
- Alam HB, Chen Z, Jaskille A, Querol RI, Koustova E, Inocencio R, Conran R, Seufert A, Ariaban N, Toruno K, Rhee P. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in Swine. J Trauma 2004;56(5):974-83.
- Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma 2006;60(3):655-8.
- Brown MA, Daya MR, Worley JA. Experience with chitosan dressings in a civilian EMS system. J Emerg Med 2009;37(1):1-7.
- Tsai GJ, Su WH. Antibacterial activity of shrimp chitosan against Escherichia coli. J Food Prot 1999;62(3):239-43.
- Pusateri AE, McCarthy SJ, Gregory KW, Harris RA, Cardenas L, McManus AT, Goodwin CW, Jr. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma 2003;54(1):177-82.
- Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressin: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med 2008;15(1):74-81.
- Q He et al. Positive charge of chitosan retards blood coagulation on chitosan films. J Biomaterial Applications. 27 (8): 1032-1045, May 2013.